27
Th4
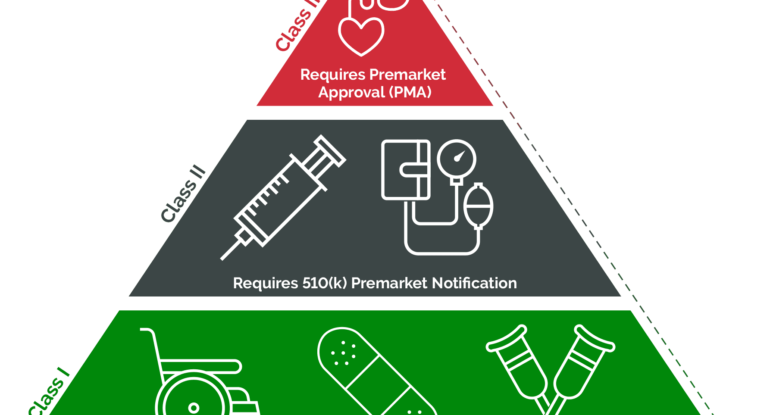
Explaining the Differences of the 3 FDA Medical Device Classes
Success in conquering the U.S. medical device market requires a clear understanding of the FDA classification system. In this article, GOL will provide you with knowledge about the differences of FDA medical device classes: Class I, Class II, and Class III, along with the regulatory requirements and market approval pathways for each type. What are […]

29
Th3
What is the frequency of medical device audits by the FDA?
In preparation for an FDA medical device audit, the manufacturer should have a plan in place to identify the necessary documents and records, as well as train employees on how to answer the inspector’s questions. During the audit, the manufacturer should fully cooperate with the FDA inspector and provide them with all necessary information. If […]

04
Th3
What is Premarket Approval (PMA)? – The difference between FDA (510k) and PMA
When launching a new medical device to the market, manufacturers must comply with the strict regulations of the U.S. Food and Drug Administration (FDA). The two most common processes are PMA (Premarket Approval) and 510(k). In this article, GOL will explain what PMA is, compare it to 510(k), and help you understand the appropriate process […]

16
Th1
What is FDA 510(k)? Learn about the FDA 510(k) certification
The United States is one of the largest medical device markets globally. To be authorized to market medical devices in this industry, manufacturers must comply with the regulations of the Food and Drug Administration (FDA). One of the crucial regulations is the FDA 510(k) certification. So, what is FDA 510(k)? What are the regulations associated […]

16
Th1
What is a FDA medical device? Overview of Medical Device Classification
Medical devices are among the products that directly impact the health and well-being of users. Therefore, ensuring the quality and safety of medical devices is of utmost importance.In the United States, the Food and Drug Administration (FDA) is the authorized agency responsible for managing and overseeing the quality of medical devices. The FDA has established […]

