27
Th4
Explaining the Differences of the 3 FDA Medical Device Classes
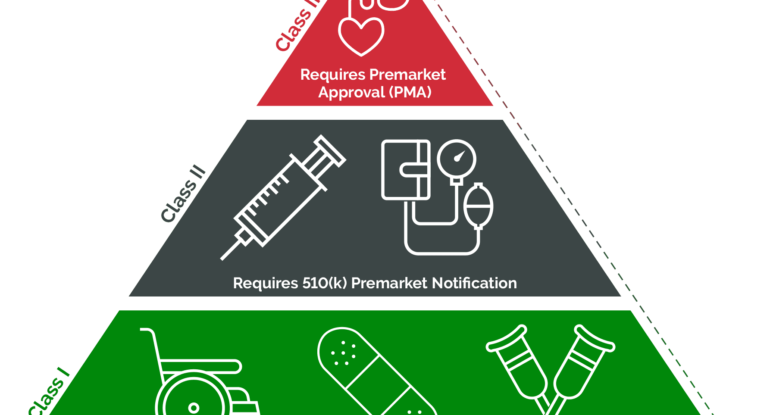
Success in conquering the U.S. medical device market requires a clear understanding of the FDA classification system. In this article, GOL will provide you with knowledge about the differences of FDA medical device classes: Class I, Class II, and Class III, along with the regulatory requirements and market approval pathways for each type. What are […]
26
Th4
What is FDA QSR? Differences between FDA QSR and ISO 13485
In the realm of medical device manufacturing, compliance with regulatory standards is paramount. Two key frameworks governing quality management are the Food and Drug Administration Quality System Regulation (FDA QSR) and the International Organization for Standardization (ISO) 13485. While both aim to ensure product safety and quality, they differ in scope, certification processes, and global […]

26
Th4
What is FDA 483? Important Details About FDA 483 Forms
The FDA 483 form plays a crucial role in safeguarding consumer health by ensuring that products manufactured and circulated in the US market meet strict quality and safety standards. In this article, GOL will provide you with important information about the FDA 483 form, including its contents, issuance process, response methods, and consequences of non-compliance. […]

26
Th4
What is ultra-processed food? How does it affect health?
In today’s modern food landscape, the term “ultra-processed food” has become increasingly prevalent, sparking discussions and debates about its impact on health. As we navigate through aisles lined with brightly colored packaging and seemingly endless options, it’s crucial to understand what exactly constitutes ultra-processed food and the implications it holds for our well-being. From convenience […]

26
Th4
The process of registering medical devices with the FDA
The process of registering medical devices with the FDA is a crucial step for companies looking to bring their products to market in the United States. With a healthcare landscape governed by stringent regulations and evolving standards, understanding and successfully navigating the FDA registration process is essential for ensuring compliance and facilitating market access. From […]

25
Th4
What You Need to Know About Food Color Additives
Food color additives are everywhere, brightening everything from candies to beverages. But what exactly are these substances, and why are they used? Are they safe? In this article, we’ll explore the essentials about these additives — their types, uses, safety, and the regulations that ensure they meet strict standards. Let’s dive into the vivid world […]

24
Th4
How to renew FDA registration? Details on time and cost
Understanding the ins and outs of renewing FDA registration can be daunting for businesses in regulated industries like food, drugs, and medical devices, given the strict deadlines, regulatory requirements, and potential fees involved, all of which are essential for maintaining compliance and ensuring operational continuity. In this article, GOL will delve into the intricacies of […]

01
Th4
What are the consequences of food mislabeling products?
Mislabeling of food products can have far-reaching consequences, impacting both consumers and businesses alike. In today’s highly regulated food industry, accurate labeling is not just a matter of compliance but also a critical aspect of ensuring public health and safety. From allergen exposure to legal liabilities, the repercussions of mislabeling can be severe. This article, […]




