
10
Th5
Free FEI Number Registration: Expedite your entry into the US market by acquiring your “key” to success.
FEI Number Registration plays a crucial role in exporting cosmetics to the United States market. Akin to a gateway key, this number is a mandatory requirement for businesses to register FDA cosmetics and list their products. This article delves into the details of FEI Number, encompassing its definition, significance, registration and lookup procedures, and the […]

09
Th5
Understanding risk assessment for food safety
Food safety risk assessment is a vital tool for protecting public health. It helps identify, assess, and control potential hazards in the food supply chain, preventing foodborne illnesses before they occur. As a result, ensuring food safety enhances quality and builds consumer trust. In this article, GOL will provide real-life examples of applying food safety […]

09
Th5
A Guide to Drug Master Files
Among the crucial steps towards FDA approval lies the creation of a Drug Master File (DMF), a comprehensive document that serves as a repository of vital information pertaining to a drug product. This detailed dossier encompasses intricate details about manufacturing facilities, production processes, active ingredients, packaging materials, and other critical aspects of the drug’s journey […]

27
Th4
FDA regulations on color additives used in the US
Color additives play a crucial role in capturing consumer attention and making food, pharmaceuticals, cosmetics, and medical devices more appealing. However, the use of color additives in these products must adhere to strict regulations set forth by the United States Food and Drug Administration (FDA) to ensure consumer safety. In this article, GOL will provide […]
27
Th4
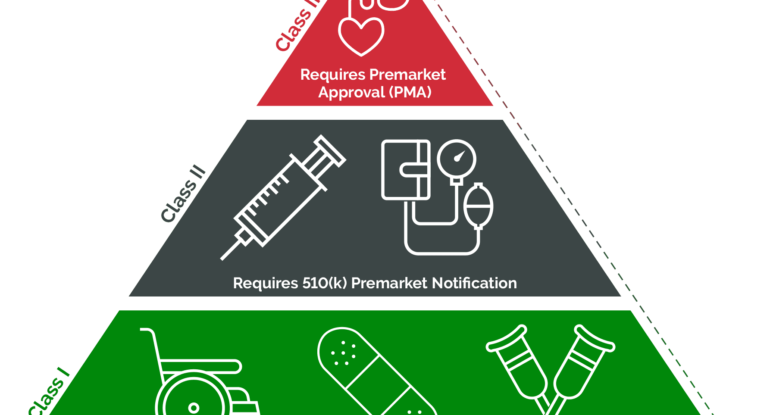
Explaining the Differences of the 3 FDA Medical Device Classes
Success in conquering the U.S. medical device market requires a clear understanding of the FDA classification system. In this article, GOL will provide you with knowledge about the differences of FDA medical device classes: Class I, Class II, and Class III, along with the regulatory requirements and market approval pathways for each type. What are […]
26
Th4
What is FDA QSR? Differences between FDA QSR and ISO 13485
In the realm of medical device manufacturing, compliance with regulatory standards is paramount. Two key frameworks governing quality management are the Food and Drug Administration Quality System Regulation (FDA QSR) and the International Organization for Standardization (ISO) 13485. While both aim to ensure product safety and quality, they differ in scope, certification processes, and global […]

26
Th4
What is FDA 483? Important Details About FDA 483 Forms
The FDA 483 form plays a crucial role in safeguarding consumer health by ensuring that products manufactured and circulated in the US market meet strict quality and safety standards. In this article, GOL will provide you with important information about the FDA 483 form, including its contents, issuance process, response methods, and consequences of non-compliance. […]

26
Th4
What is ultra-processed food? How does it affect health?
In today’s modern food landscape, the term “ultra-processed food” has become increasingly prevalent, sparking discussions and debates about its impact on health. As we navigate through aisles lined with brightly colored packaging and seemingly endless options, it’s crucial to understand what exactly constitutes ultra-processed food and the implications it holds for our well-being. From convenience […]



